Guided by brand strategy
Based on product quality

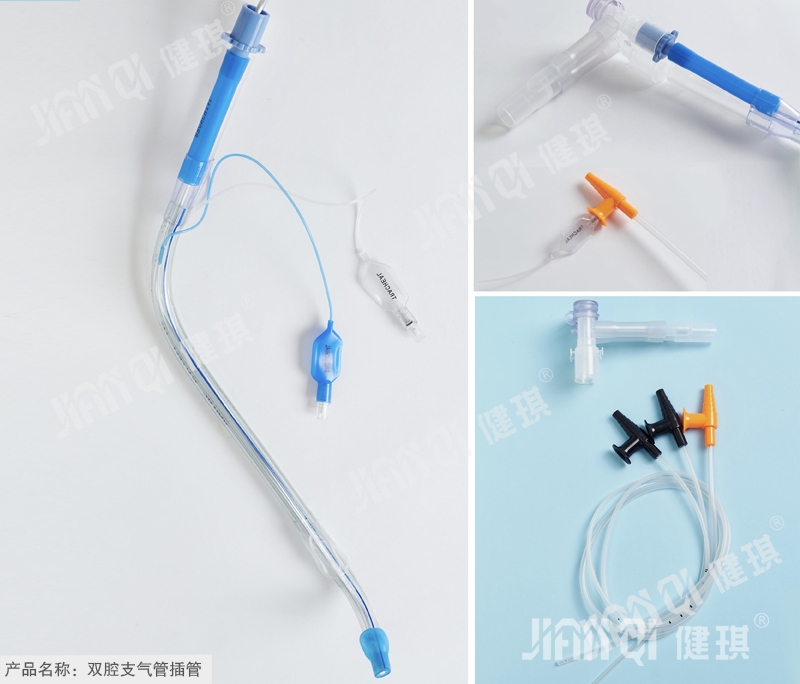
[product name] double lumen endobronchial intubation
[specifications and models] fr26, fr28, fr31, fr32, fr33, fr35, fr37, FR39, fr41
[name of registrant] Henan Jianqi medical device Co., Ltd
[residence] dingluan Industrial Zone, Changyuan County
[Tel.] 0373-8690789
[after sales service unit] Henan Jianqi medical equipment sales Co., Ltd
[name of manufacturer] Henan Jianqi Medical Equipment Co., Ltd
[residence] dingluan Industrial Zone, Changyuan County
[production address] dingluan Industrial Zone, Changyuan County
[contact information] 0373-8690789
[production license] ysyjx production license No. 20080036
[Registration Certificate No.] yxzz 20162660347
[technical requirements No.] yxzz 20162660347
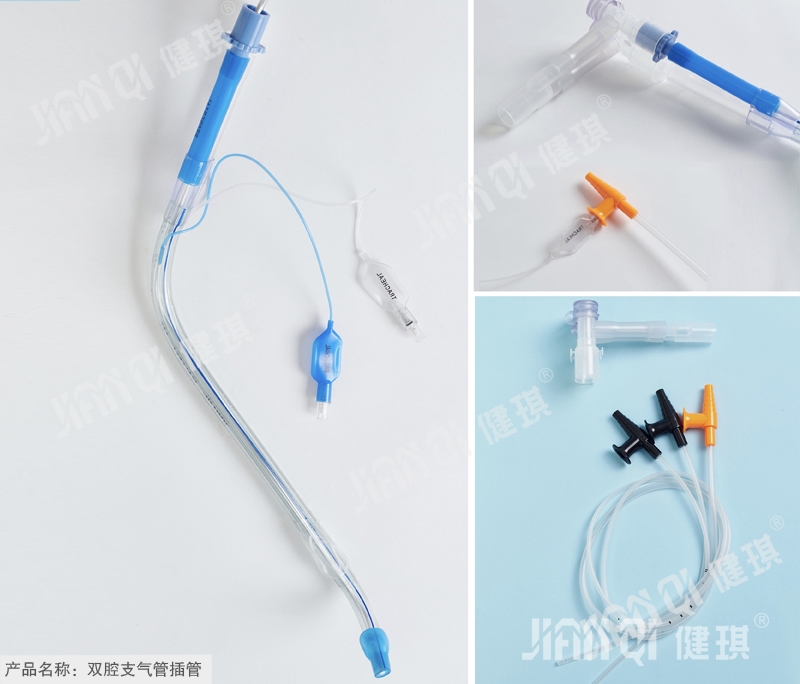
[structure and composition] it is composed of cuff, indicator bag, check valve, inflation tube, joint and tube body.
[product performance] 1. The head of the guide core inserted into the tube body shall be round and blunt, and the tube body shall be smooth; all components of the double chamber tube shall be free from obvious mechanical impurities or molding defects; the end and inclined plane of the patient end of the double chamber tube shall be smooth. There should be no burr in the Murphy hole; the mark of the double chamber tube should be printed clearly, indicating that the air bag should be consistent with the printing on the tube body. 2. Right type double lumen tube: the cutting surface of the large cavity is 70mm ± 0.5mm from the patient end of the double lumen tube, and the bending radian of the machine end is 135 °± 5 °; there should be a Murphy hole on the double lumen tube, and the distance between the front end of the Murphy hole and the patient end is 25 mm ± 0.5mm; the distance between the front edge of the first balloon of the double lumen tube and the inclined plane of the end of the double lumen patient is 1 The distance between the edge of the effective part of the second spherical capsule and the back edge of the Murphy hole should not be more than 1 mm ± 0.1 mm. The distance between the front edge of the sleeve of the cylindrical capsule and the part near the machine end of the inclined plane is 1.5 mm ± 0.5 mm. Left type double lumen tube: the cutting surface of large cavity is 60mm ± 0.5mm away from the end face of patient end of double lumen tube, and the bending radian of machine end is 135 °± 5 °. The distance between the front edge of spherical capsule sleeve and the end of patient end is 1.5 mm ± 0.5mm, and the distance between the end of cylindrical capsule sleeve and the edge of inclined plane close to machine end is 1.5 mm ± 0.5mm. 3. The cuff (spherical, cylindrical and indicator balloon) should be able to withstand a positive pressure of 3kpa ± 0.2kpa without rupture. 4. The color of PVC flexible joint shall be the same as that of the sleeve in the closed system connected to it; the length of the joint shall be 80mm ± 2mm. 5. The length of inflation tube should be more than 250mm ± 2mm. 6. The gas valve injection port shall meet the requirements of 6:100 semi-rigid conical joint specified in GB 1962.1-2001; the air valve shall not have air leakage. 7. The machine end of the connecting pipe shall be a conical joint with an outer diameter of 15mm at the small end. 8. The cross, soft joint and pipe body of double chamber pipe shall be firmly combined. 9. The double chamber tube inflation system shall have good sealing performance, and the bonding parts (inflation tube and double chamber tube, valve bag and inflation tube, sleeve bag and double chamber tube) shall not leak. 10. The total metal content and cadmium content in the test solution of double chamber tube shall not exceed the product standard requirements. 11. The pH value of the product should be 6.5-7.5. 12. The residual amount of ethylene oxide should not be more than 10g / UG. 13. The product should be sterile.
[scope of application] it is suitable for thoracic and vascular surgery, critical patients when one lung is independent, and when synchronous or asynchronous ventilation is used.
[contraindications] 1. Patients with laryngeal edema, acute laryngitis, submucosal edema of larynx and severe bleeding caused by intubation trauma.
2. The patients with respiratory tract compression and trachea obstruction were not found.
[precautions] 1. This product is disposable and its package is damaged. It is forbidden to use it and destroy it after use.
2. The production batch number is shown in the seal or certificate, and the sterilization date is shown in the outer packing box.
3. The product was sterilized by ethylene oxide.
[method of use] 1. Select the specification and model of the patient, make sure that the outer package is in good condition, open the package and take out the catheter.
2. Make the patient lie on his back and check the oral cavity (take out the foreign body and removable denture, no falling tongue).
3. Using laryngoscope to lift the epiglottis to expose glottis and insert organ cannula along the side of laryngoscope.
4. The success of intubation can be tested by auscultation, sputum suction tube patency test and fiberoptic bronchoscopy.
5. After the end of use, the product should be treated as medical waste to avoid pollution.
[storage method] 1. The product should be loaded and transported in covered carriage and cabin, and kept clean. It should not be under heavy pressure, direct sunlight, rain or snow.
2. Handle with care to avoid violent collision;
3. The product should be stored away from the fire source, the relative humidity is not more than 80%, no corrosive gas and well ventilated and clean environment.

| Contact person: Ms Jin | Foreign trade: Carol Qiu |
| Phone:+86 13938450051 | |
| Landline:+86 0371 67112186 | |
 hnjq2008@163.com hnjq2008@163.com | sales@hnjianqi.com |
 www.hnjianqi.com www.hnjianqi.com | www.jianqimedical.com |
Zone, Changyuan City, Henan |