Guided by brand strategy
Based on product quality

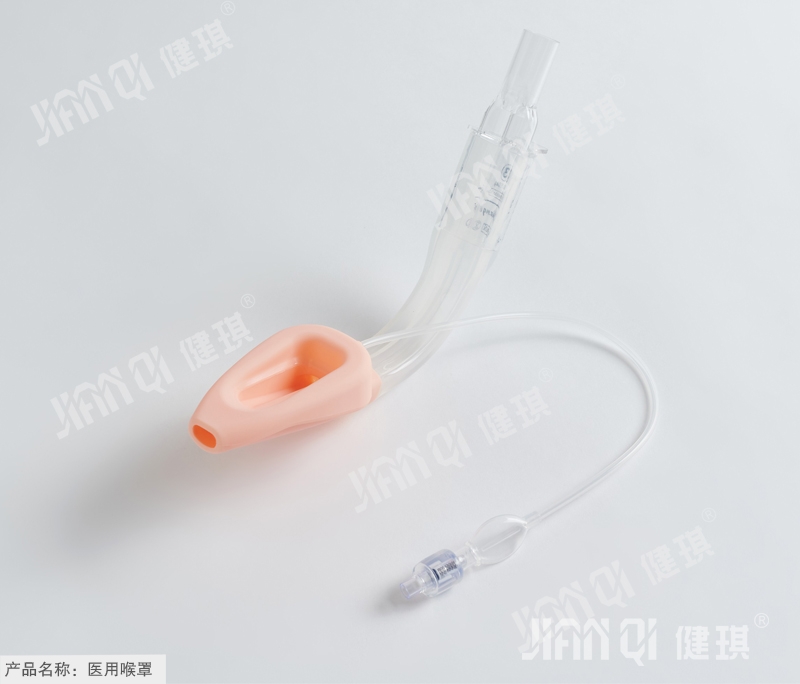
[product name] medical laryngeal mask
[specification and model] model: inflatable type can be divided into single cavity common type, single cavity reinforced type, double cavity common type and double cavity reinforced type;
The gas free type is double chamber type, with specifications of 1.0 mm, 1.5 mm, 2.0 mm, 2.5 mm, 3.0 mm, 4.0 mm, 5.0 mm.
[name of registrant] Henan Jianqi medical device Co., Ltd
[address] dingluan Industrial Zone, Changyuan County, Henan Province
[Tel: 0373-8690789]
[after sales unit] Henan Jianqi medical equipment sales Co., Ltd
[name of manufacturer] Henan Jianqi Medical Equipment Co., Ltd
[address] dingluan I Industrial Zone, Changyuan County, Henan Province
[production address] dingluan Industrial Zone, Changyuan County, Henan Province
[contact information] 0373-8690789
[production license] ysyjx production license No. 20080036
[Registration Certificate No.] yxzz 20182140534
Laryngeal mask for medical use (dual chamber common type). Jpg
[main structure and performance] this product is composed of ventilation hood, inflation tube, vent pipe, joint inflation valve and tube body. Laryngeal mask has less irritation, less cardiovascular reaction when inserting and pulling out, less throat pain after operation
Damage to the airway.
Main performance: 1. Joint: the machine end of the joint should be a 1 5mm outer cone joint, and the size should conform to the provisions of yy1040.1 -- 2003 standard.
2. Check valve: the check valve shall meet the requirements of 6% (Ruhr) inner cone joint specified in GB / t1962.1-2001.
3. Ventilation hood: inclined angle of 38 ° + 10 ° with pipe axis.
4. Connection firmness: the connection between the joint and the main pipe, the air pipe and the air bag, the inflation pipe and the indicating air bag, and the main pipe and the connecting pieces shall be firmly connected. Under the action of 15N axial static tension respectively applied at each joint, it lasts for 15s
Fall off or separate.
5. Tightness: the joints of laryngeal mask airway, main pipe and gas device shall be well sealed. Under the positive pressure of 3kpa + 0.2kpa, each bonding part shall be free of air leakage.
6. Ethylene oxide residue: if ethylene oxide is used for sterilization, the residual amount shall not be greater than 10 μ g / g.
7. Biological properties
The laryngeal mask should be sterile, and the sterile period of validity is two or two years. There should be no delayed hypersensitivity in LMA. 7.3. The cytotoxicity of LMA should be no more than grade 2. 7.4 laryngeal mask should have no intradermal stimulation.
8. PH: pH value of LMA should be 6.5 ~ 7.5.
[scope of application] for general anesthesia or cardiopulmonary resuscitation to establish short-term artificial airway.
[contraindications] 1. This product is mainly made of medical silica gel material, which is prohibited for allergic patients;
2. Those who are full of food, not fasting and have reflux risk should use it with caution.
3. It is forbidden for patients with tracheal compression and softening who have respiratory obstruction after anesthesia.
4. The patients who need positive pressure ventilation should be cautious when they have airway obstruction, decreased lung compliance or high respiratory resistance caused by throat diseases.
5. Patients with laryngeal edema, acute inflammation of respiratory tract and throat abscess should be used with caution.
[note] 1. This product is disposable, the package is damaged, it is strictly prohibited to use, and then destroyed.
After sterilization, the product is sterile for two years.
3. The production batch number and expiration date are shown in the seal of the package, and the sterilization date is shown in the outer packing box.
4. During the use of LMA, attention should be paid to tracheal or pulmonary reflux aspiration.
5. If the patient's head or neck changes with the position when the LMA is inserted, it is necessary to confirm again whether the LMA is unblocked, and then use it again.

| Contact person: Ms Jin | Foreign trade: Carol Qiu |
| Phone:+86 13938450051 | |
| Landline:+86 0371 67112186 | |
 hnjq2008@163.com hnjq2008@163.com | sales@hnjianqi.com |
 www.hnjianqi.com www.hnjianqi.com | www.jianqimedical.com |
Zone, Changyuan City, Henan |