Guided by brand strategy
Based on product quality

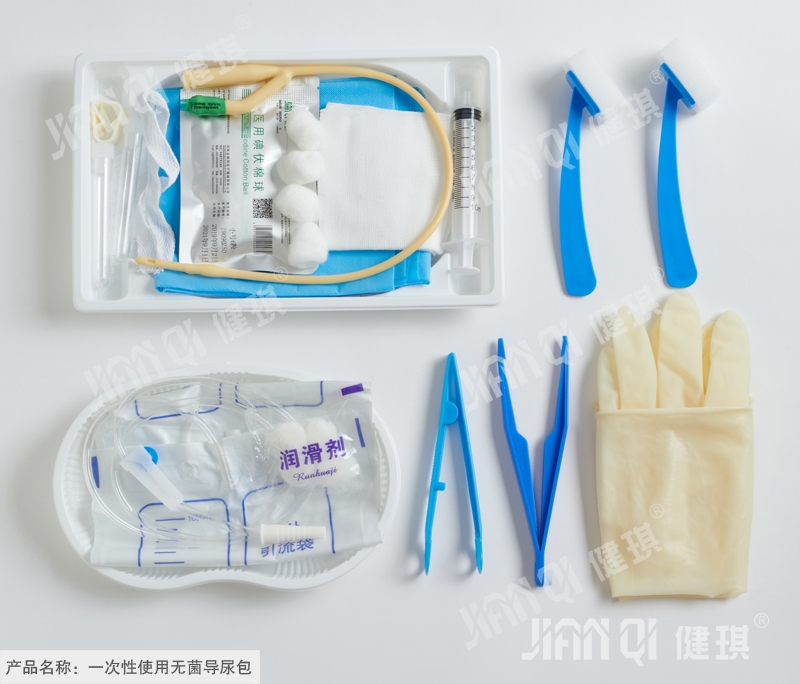
[product name] disposable sterile catheterization kit
[specification and model] model: single cavity, double chamber, three cavity; specification: fr6, fr8, fr10, fr12, fr14, fr16, FR18, fr20, fr22, fr24, fr26, fr28, FR30;
[name of registrant] Henan Jianqi medical device Co., Ltd
[residence] dingluan Industrial Zone, Changyuan County
[Tel.] 0373-8690789
[after sales service unit] Henan Jianqi medical equipment sales Co., Ltd
[name of manufacturer] Henan Jianqi Medical Equipment Co., Ltd
[residence] dingluan Industrial Zone, Changyuan County
[production address] dingluan Industrial Zone, Changyuan County
[contact information] 0373-8690789
[production license] ysyjx production license No. 20080036
[Registration Certificate No.] yxzz 20172660081
[technical requirement No.] yxzz 20172660081
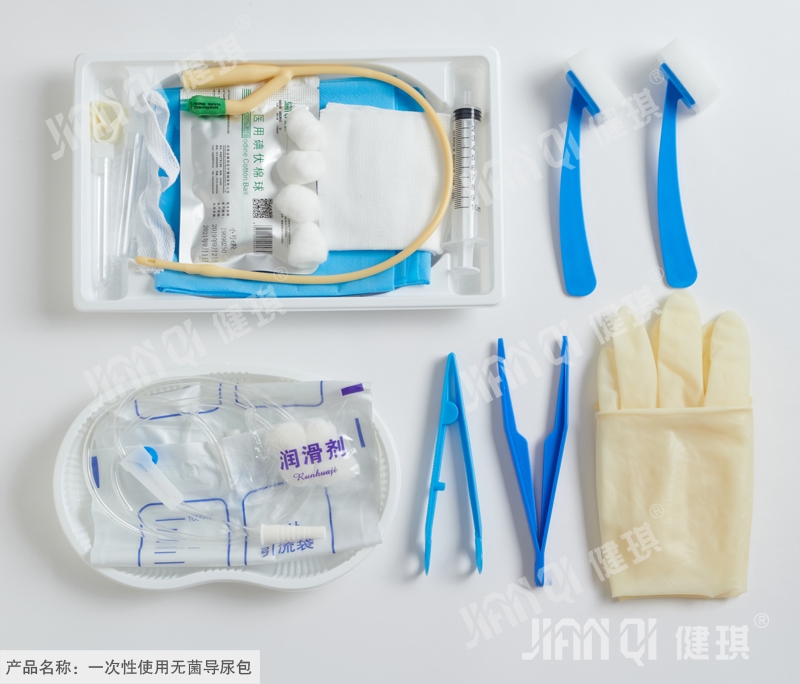
[structure and composition] this product consists of basic configuration and optional configuration. Basic configuration: disposable sterile catheter, disposable drainage bag, medical tweezers, medical cotton ball and optional configuration: disposable medical rubber inspection gloves, iodophor cotton ball, paraffin cotton ball, medical degreasing gauze block, disposable hole towel, goods box, cloth, sterile water test tube, disposable dispensing syringe, sponge brush, disposable sterilization Rubber surgical gloves and PE inspection gloves.
[product performance] 1. The package of catheterization bag should be well sealed without cracks and holes; the accessories in the bag should be free from damage, stains and impurities. 2. The body, tip, balloon and eyelet of disposable sterile catheter should be free of foreign substances; the nominal outer diameter of catheter should be 0.01mm, and the tolerance should be ± 0.33mm. The volume of the balloon should be expressed in ml; the total length (L) and the length of the tube body (s) should be in accordance with table 1 (see also figure 1); the tip and conical interface should be integrated with the pipe body, and the pipe body should not be broken; the discharge cone interface should not be separated from the test connector; the balloon should not leak and should not affect the drain hole; when tested according to Appendix D of yy0325-2002 standard, the water recovery rate should not be lower than that in Appendix D The values specified in table D.2; the flow rate shall be in accordance with table 2. 3. Disposable drainage bags should be qualified products with medical device registration certificate. 4. Tweezers should be symmetrical, and the appearance should be smooth without sharp edges, burrs, cracks, pockmarks and trachoma. The tweezers should have good elasticity; the two pieces of tweezers should be firmly connected, and should be able to bear 15N tension without fracture; the lip teeth should be clear and complete without missing or rotten teeth; the guide pin and positioning pin of the tweezers should be fixed firmly, flexible when the tweezers are opened and closed, and there should be no jam phenomenon; the handle flowers of tweezers should be clear and complete without missing or rotten flowers. 5. Medical cotton ball should be a qualified product with medical device registration certificate. 6. Disposable medical rubber inspection gloves should be qualified products with medical device registration certificate. 7. It should be a qualified product with medical device registration certificate. 8. Iodophor cotton ball should be a qualified product with medical device registration certificate. 9. Paraffin tampons should be qualified products with medical device registration certificate. 10. Medical absorbent gauze should be qualified products with medical device registration certificate. 11. Disposable pore towel should be a qualified product with medical device registration certificate. 12. The appearance of the box should be smooth, without burr or obvious deformation; the thickness should not be less than 0.01mm. 13. The longitudinal breaking strength of nonwovens used for cloth laying shall be greater than or equal to 13N, and the transverse breaking strength shall be greater than or equal to 8N; the surface of the nonwovens shall be clean and free from stains, and the cutting around shall be neat and free of burrs; the specification of nonwovens used shall not be less than 20g / m2. 14. The water for sterilization is 10ml / piece, which is in line with the provisions in the second part of Pharmacopoeia of the people's Republic of China (2010 Edition). 15. Disposable dispensing syringes should be qualified products with medical device registration certificate. 16. The adhesive force between the sponge head and the brush shall not be less than 10N, and the joint shall not be loose. 17. Disposable sterilized rubber surgical gloves should be qualified products with medical device registration certificate. 18. PE inspection gloves should be qualified products with medical device registration certificate. 19. The catheterization bag should be sterile; 20. The residual amount of ethylene oxide should not be greater than 10ug / G after sterilization with ethylene oxide.
[scope of application] it is applicable to patients undergoing catheterization.
[contraindications] no contraindications.
[precautions] 1. This product is disposable and its package is damaged. It is forbidden to use it and destroy it after use.
2. See the seal or label of the package for the batch number and the outer packing box for the sterilization date.
3. The product was sterilized by ethylene oxide.
[usage] 1. Open the package after confirming that the outer package is in good condition.
2. Take out the accessories in the bag according to the demand.
3. After the end of use, the product should be treated as medical waste to avoid pollution.
[storage method] 1. The product should be loaded and transported in covered carriage and cabin, and kept clean. It should not be under heavy pressure, direct sunlight, rain or snow.
2. Handle with care to avoid violent collision;
3. The product should be stored away from the fire source, the relative humidity is not more than 80%, no corrosive gas and well ventilated and clean environment.

| Contact person: Ms Jin | Foreign trade: Carol Qiu |
| Phone:+86 13938450051 | |
| Landline:+86 0371 67112186 | |
 hnjq2008@163.com hnjq2008@163.com | sales@hnjianqi.com |
 www.hnjianqi.com www.hnjianqi.com | www.jianqimedical.com |
Zone, Changyuan City, Henan |