Guided by brand strategy
Based on product quality

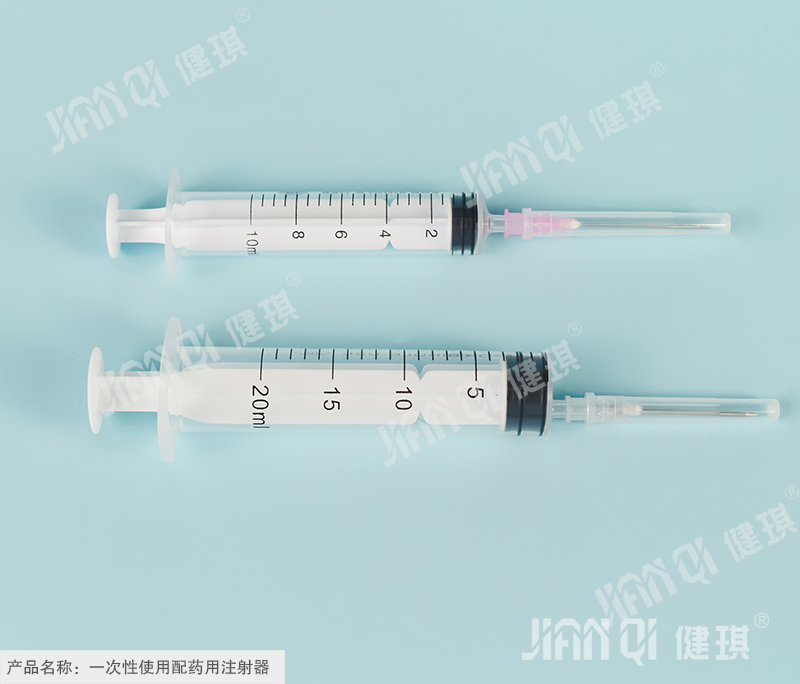
[product name] disposable dispensing syringe
[specification and model] according to the structure, it is divided into two pieces and three pieces; according to the type, it is divided into middle head type and side head type; according to the capacity, it is divided into 10ml, 20ml, 30ml, 50ml and 100ml. According to the needle tip, the dispensing needle can be divided into side hole needle (CZ) and inclined plane needle (XZ); thin wall (TW) and normal wall (RW) according to the needle tip; according to the outer diameter, it can be divided into Φ 0.9mm, Φ 1.2mm, Φ 1.4mm, Φ 1.6mm, Φ 1.8mm, Φ 2.1mm, Φ 2.4mm, Φ 2.7mm, Φ 3.0mm and Φ 3.4mm.
[name of registrant] Henan Jianqi medical device Co., Ltd
[residence] dingluan Industrial Zone, Changyuan County
[Tel.] 0373-8690789
[after sales service unit] Henan Jianqi medical equipment sales Co., Ltd
[name of manufacturer] Henan Jianqi Medical Equipment Co., Ltd
[residence] dingluan Industrial Zone, Changyuan County
[production address] dingluan Industrial Zone, Changyuan County
[contact information] 0373-8690789
[production license] ysyjx production license No. 20080036
[Registration Certificate No.] yxzz 20182140496
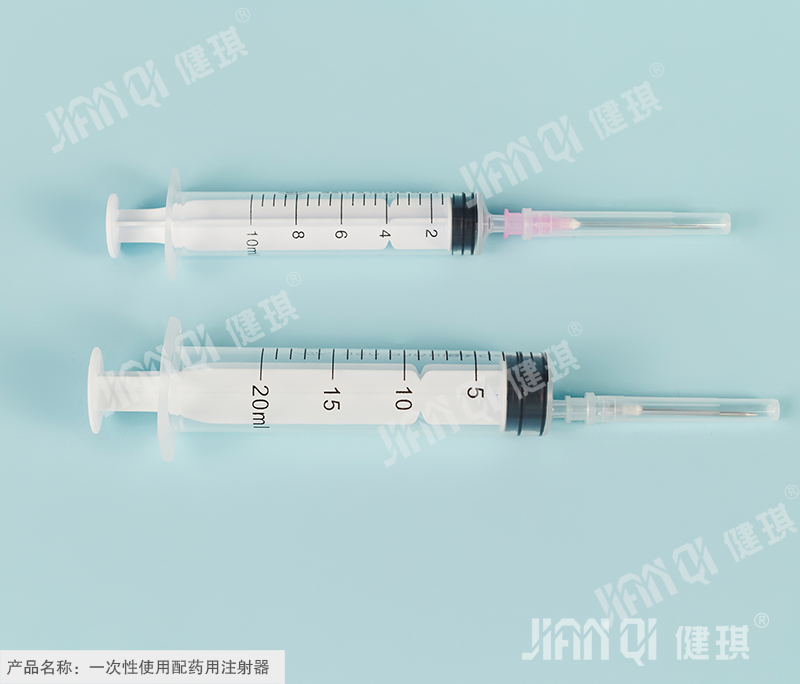
[structure and composition] it is composed of dispensing device and dispensing needle. The dispensing device is composed of a ruler, a zero scale line, a dividing capacity line, a nominal capacity scale line, an outer sleeve curling edge, a cone head, an outer sleeve, a piston, a core rod and a pressing hand; a dispensing needle is composed of a needle base, a needle tube and a sheath.
[product performance] 1 the outer cone joint of cone head shall comply with the provisions of GB / T 1962.1-2001 or GB / T 1962.2-2001. 2. Under the water pressure of no more than 100KPA, the flow rate of the side hole needle tube should not be less than 80% of the needle tube with the same outer diameter, length and inner diameter under the same conditions. 3. The contamination index of the side hole needle should not exceed 90. 4. More than 3 chips should be produced per 100 times of puncture. 5. The static axial force specified in Table 1 shall be applied at the joint of needle tube and needle base for 15s, and the two shall not be loose or separated. 6. There shall be no corrosion marks on the immersed part of the needle tube. The taper joint of 7 needle holder shall comply with the provisions of GB / T 1962.1-2001 or GB / T 1962.2-2001. 8. During the negative pressure test, there should be no air leakage at the contact part between the jacket and the piston, and the piston and the core rod should not be separated. 9. The tolerance of the dispensing device's capacity is equal to or greater than half of the nominal capacity, and the tolerance should comply with the relevant provisions in Table 1. 10. The volume of liquid remaining in the outer sleeve of the dispenser shall not exceed that specified in Table 1. 11. The total content of lead, zinc, tin and iron in the leaching solution of dispensing device should be less than 5 μ g / ml, and the content of cadmium should be less than or equal to 0.1 μ g / ml. 12. The pH value difference between the solution extracted from the dispenser and the blank control solution of the same batch shall not exceed 1.0. 13. The difference of the consumption of 0.002mol/l potassium permanganate solution should be 0.5ml when compared with the same volume of blank control solution of the same batch. 14. After sterilization with ethylene oxide, the residual amount of ethylene oxide is no more than 10 μ g / g. 15. The dispenser is sterile.
[scope of application] it is used for clinical extraction, dissolution and preparation.
[contraindications] no contraindications.
[precautions] 1. This product is disposable and its package is damaged. It is forbidden to use it and destroy it after use.
2. The production batch number and expiration date are shown in the seal or certificate of quality, and the sterilization date is shown in the outer packing box.
3. This product is sterilized by ethylene oxide and has a sterility validity of two years.
4. No human injections.
5. It can be used as soon as it is ready to use. It should not be retained and destroyed after use.
6. It is forbidden to touch the liquid medicine or dispensing needle with hands or non sterilized articles during operation.
7. When the side hole needle is used, the diluent should be aspirated many times, and all the diluents should be used for the patients.
8. It is not applicable to polyolefin plastic injection molding equipment which has been indicated in the drug instructions.
9. It should be used with caution when allergic to polyolefin plastics and stainless steel materials.
[usage] 1. Take the product out of the small package and connect the syringe cone head with the dispensing needle of the required specification;
2. Remove the protective cover of the dispensing injection needle and insert the dispensing injection needle into the drug;
3. Pull the core rod to suck the liquid into the outer sleeve of the dispensing syringe;
4. After exhausting the air in the syringe jacket, the medicine solution can be prepared;
5. After dispensing, put the product in the recycling position for unified treatment.
[storage method] 1. The product should be loaded and transported in covered carriage and cabin, and kept clean. It should not be under heavy pressure, direct sunlight, rain or snow.
2. Handle with care to avoid violent collision;
3. The product should be stored away from the fire source, the relative humidity is not more than 80%, no corrosive gas and well ventilated and clean environment.

| Contact person: Ms Jin | Foreign trade: Carol Qiu |
| Phone:+86 13938450051 | |
| Landline:+86 0371 67112186 | |
 hnjq2008@163.com hnjq2008@163.com | sales@hnjianqi.com |
 www.hnjianqi.com www.hnjianqi.com | www.jianqimedical.com |
Zone, Changyuan City, Henan |