Guided by brand strategy
Based on product quality

[product name] disposable blood collection kit
[specification and model] type I and type II.
[name of registrant] Henan Jianqi medical device Co., Ltd
[residence] dingluan Industrial Zone, Changyuan County
[Tel.] 0373-8690789
[after sales service unit] Henan Jianqi medical equipment sales Co., Ltd
[name of manufacturer] Henan Jianqi Medical Equipment Co., Ltd
[residence] dingluan Industrial Zone, Changyuan County
[production address] dingluan Industrial Zone, Changyuan County
[contact information] 0373-8690789
[production license] ysyjx production license No. 20080036
[Registration Certificate No.] yxzz 20142640124
[technical requirements No.] yxzz 20142640124
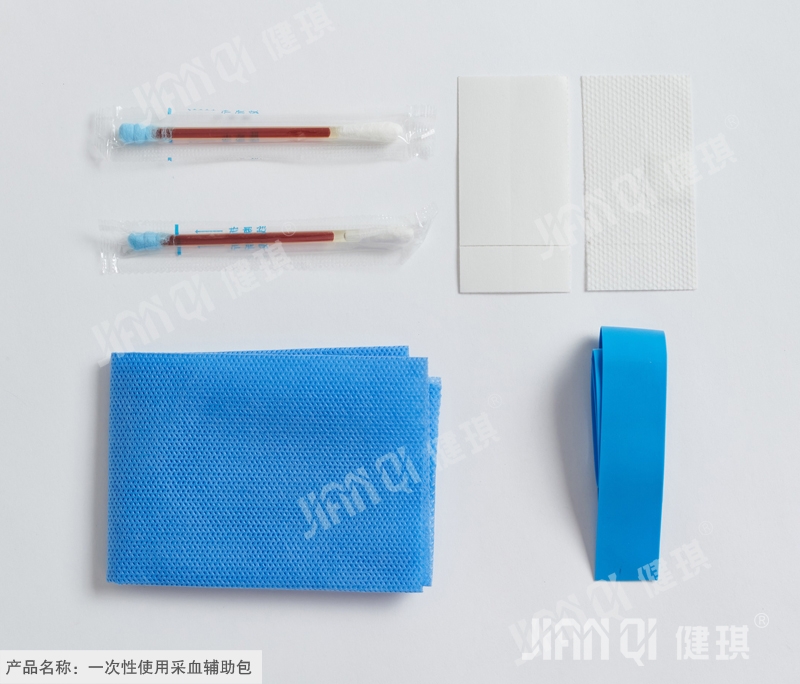
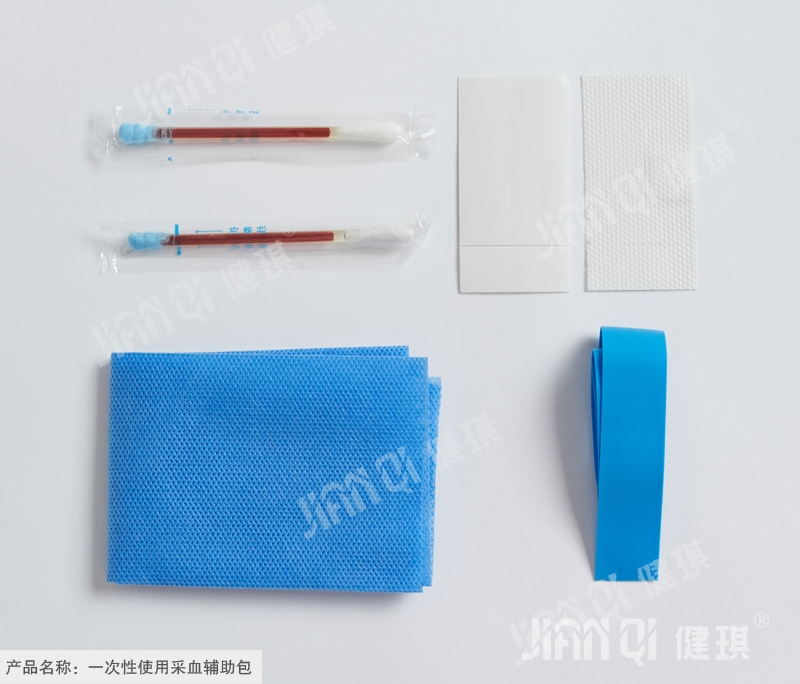
[structure and composition] the product is composed of basic configuration and optional configuration; type I basic configuration: medical cotton swab and medical infusion stick; type II basic configuration: medical cotton swab and tray; type I and type II selection and configuration include: medical cotton ball, hemostatic tape, medical bag, disposable treatment towel, PE inspection gloves, disposable medium single, iodophor cotton swab, iodophor cotton ball and breathable adhesive The utility model is composed of a belt, a medical forceps and a tray.
[product performance] 1. The name, specification and quantity of the items configured in the blood collection auxiliary package shall meet the requirements in Table 1 and table 2 of the product technical requirements. 2. The auxiliary blood collection package should be well sealed without gaps and holes; the accessories in the blood collection auxiliary bag should be free from damage, stain and impurity; the folding parts should be folded neatly. 3. The specification and size of medical cotton swab and the weight of cotton swab head are specified in Table 3 of product technical requirements. 4. The surface of the cotton head shall be clean and free of stains and impurities, and the surface of bamboo stick shall be smooth and free of burr; the bamboo stick and the tail of the cotton head shall be tightly twisted and shall not fall off naturally; the appearance of the absorbent cotton shall be free of leaves, pericarp, seed coat, residue or other impurities, and there shall be certain resistance during stretching, and there shall be no dust falling off when gently shaking; Each visible fiber should be composed of a single cell with a length of 4cm and a width of 40 μ m, which is a thick round wall flat tube, usually twisted; when contacting with zinc iodide solution, the fiber should be purple; the sample should not be dissolved; only typical cotton fiber should be included, and a small amount of isolated foreign fiber should be allowed occasionally; the number of Neps in the sample should not be more than that of the standard sample; the total amount of soluble substances in the sample should not be greater than 0.50%; The results showed that the solution should not show pink; the sinking time should not exceed 10s; the water absorption per gram of medical absorbent cotton should not be less than 23.0g; the total amount of soluble substances in ether should not be more than 0.50%; the medical absorbent cotton should only have micro brown purple fluorescence and a small amount of yellow particles. In addition to a small number of isolated fibers, blue fluorescence should not be strong. The mass loss of dry weightlessness should not be greater than 8%; the ash content of sulphate should not be greater than 0.40%; surfactant foam should not cover the entire liquid surface. In a narrow and long extractor, 10.0g absorbent cotton was slowly extracted with 96% ethanol (volume fraction: 95.1% - 96.9%, about 0.81g/ml). The color of the extract should not be darker than the reference solution Y5 and GY6 specified in Appendix A or the control solution prepared according to the following methods: add 7.0ml hydrochloric acid solution (mass concentration: 10g / L) into 3.0ml primary blue solution 0.5ml of the above solution was diluted to 10.0ml with hydrochloric acid solution (10 g / L hydrochloric acid). 5. Medical infusion stickers should be qualified products with medical device registration certificate. 6 medical cotton ball should be qualified products with medical device registration certificate. The hemostatic tape should be cut neatly, the tensile length and breaking strength should be more than twice of itself, and there should be no fracture. 7 if the medical wrapping cloth is made by hot pressing, the hot pressing part shall be firm without cracking; the specification of nonwovens used shall not be less than 20g / m2; the longitudinal breaking strength shall be greater than or equal to 13N, and the transverse breaking strength shall be greater than or equal to 8N. 8 disposable treatment towel should be qualified product with medical device registration certificate. 9pe inspection gloves should be qualified products with medical device registration certificate. 10 the single use list should be the qualified product with medical device registration certificate. The iodophor cotton swabs should be qualified products with medical device registration certificate. 12 iodophor cotton ball should be a qualified product with medical device registration certificate. 13 the air permeable tape should be a qualified product with medical device registration certificate. 14. Medical tweezers should be symmetrical, smooth in appearance, free from sharp edges, burrs, cracks, pockmarks and trachoma; the tweezers should have good elasticity; the two pieces of tweezers should be firmly connected, and should be able to withstand 15N tension without fracture. The lip and teeth should be clear and complete without missing teeth or rotten teeth; The guide pin and positioning pin of tweezers shall be fixed firmly, and when the tweezers are opened and closed, they shall be flexible without jam; the handle flowers of tweezers shall be clear and complete without missing or rotten flowers. The tray should be smooth, without burr and injection flow; the thickness should not be less than 0.1 mm. 16. Aseptic blood collection kit should be sterile. The residual amount of ethylene oxide should be no more than 10 μ g / G after sterilization with ethylene oxide.
[scope of application] it is used for clinical auxiliary blood collection in medical units.
[contraindications] no contraindications.
[precautions] 1. This product is disposable and its package is damaged. It is forbidden to use it and destroy it after use.
2. See the seal or label of the package for the batch number and the outer packing box for the sterilization date.
3. The product was sterilized by ethylene oxide.
[usage] 1. Select the specifications and models of the patients, and open the package after confirming that the outer package is in good condition.
2. The accessories can be taken out according to clinical needs.
3. After the end of use, the product should be treated as medical waste to avoid pollution.
[storage method] 1. The product should be loaded and transported in covered carriage and cabin, and kept clean. It should not be under heavy pressure, direct sunlight, rain or snow.
2. Handle with care to avoid violent collision;
3. The product should be stored away from the fire source, the relative humidity is not more than 80%, no corrosive gas and well ventilated and clean environment.

| Contact person: Ms Jin | Foreign trade: Carol Qiu |
| Phone:+86 13938450051 | |
| Landline:+86 0371 67112186 | |
 hnjq2008@163.com hnjq2008@163.com | sales@hnjianqi.com |
 www.hnjianqi.com www.hnjianqi.com | www.jianqimedical.com |
Zone, Changyuan City, Henan |