Guided by brand strategy
Based on product quality

[product name] cervical cancer screening kit
[specification and model] check type
[name of registrant] Henan Jianqi medical device Co., Ltd
[address] dingluan Industrial Zone, Changyuan County, Henan Province
[Tel.] 0373-8690789
[after sales unit] Henan Jianqi medical equipment sales Co., Ltd
[name of manufacturer] Henan Jianqi Medical Equipment Co., Ltd
[address] dingluan Industrial Zone, Changyuan County, Henan Province
[production address] dingluan Industrial Zone, Changyuan County, Henan Province
[contact information] 0373-8690789
[production license] ysyjx production license No. 20080036
[Registration Certificate No.] yxzz 20182140788
[technical requirement No.] yxzz 20182140788
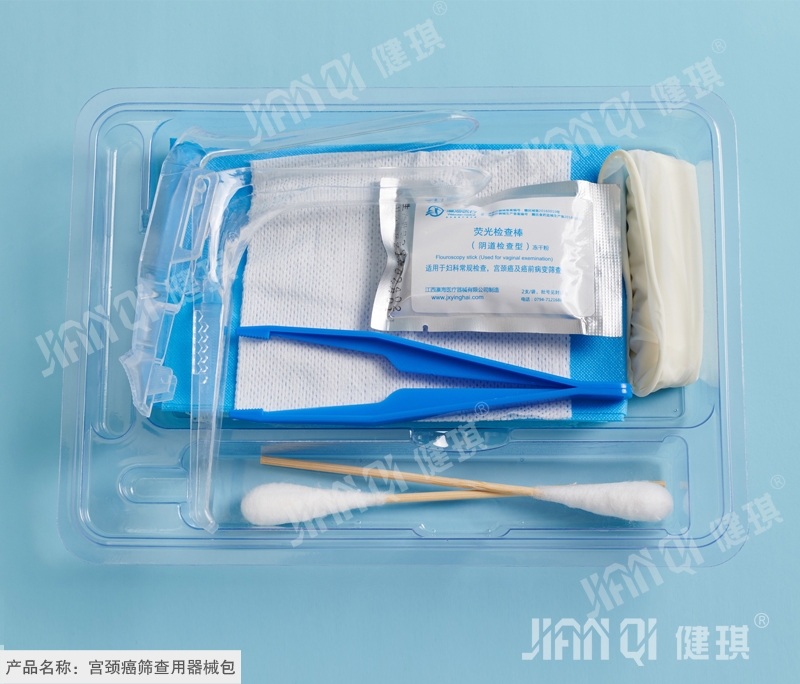
[structure and composition] basic configuration: fluorescent inspection rod, disposable medical pad, cotton swab composition: optional configuration: PE inspection gloves, disposable operating clothes, disposable medical rubber inspection gloves, disposable treatment towel, disposable single use, disposable mask, disposable hat, medical nonwoven dressing block, medical tweezers, tray, wrapping cloth It is composed of disposable sterile vaginal dilator.
[scope of application] this product is suitable for preliminary screening of cervical cancer in women.
[contraindications] no contraindications.
[precautions] 1. This product is disposable and its package is damaged. It is forbidden to use it and destroy it after use;
2. The production batch number and expiration date are shown on the seal or label of the package, and the sterilization date is shown in the outer packing box;
3. This product is for doctors and personnel who have received relevant knowledge and technology training.
[usage] 1. Open the package after confirming that the outer package is in good condition;
2. If the disposable vaginal dilator is equipped, the disposable vaginal dilator can be taken out to open the vagina; the main accessory fluorescent inspection stick is inserted into the vagina to expose the cervix and observe the discoloration of its surface;
3. Other accessories can be taken out one after another according to the needs of clinical operation;
4. After the end of use, the product should be treated as medical waste to avoid pollution.
[storage method] 1. The product shall be loaded and transported in the covered carriage and cabin, and kept clean, and shall not be exposed to heavy pressure, direct sunlight or rain and snow;
2. Handle with care to avoid violent collision;
3. The product should be stored away from the fire source, the relative humidity is not more than 80%, no corrosive gas and well ventilated and clean environment;

| Contact person: Ms Jin | Foreign trade: Carol Qiu |
| Phone:+86 13938450051 | |
| Landline:+86 0371 67112186 | |
 hnjq2008@163.com hnjq2008@163.com | sales@hnjianqi.com |
 www.hnjianqi.com www.hnjianqi.com | www.jianqimedical.com |
Zone, Changyuan City, Henan |