Guided by brand strategy
Based on product quality

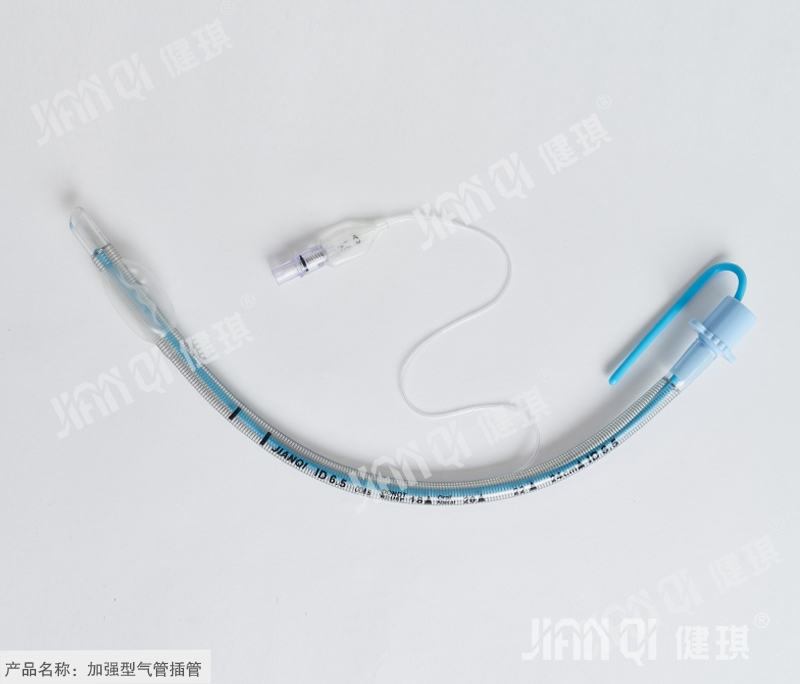
[product name] enhanced endotracheal intubation
[specification and model] 2.0, 2.5, 3.0, 3.5, 4.0, 4.0, 4.5, 4.0, 4.5, 4.5, 5.5, 5.5, 6.0, 6.5, 6.5, 7.0, 7.5, 7.5, 8.0, 8.5, 8.5, 9.0, 9.0, 9.9, 9.5, 9.5, 10.10, 10.10, 10.5, 10.5, 7.0, 7.5, 7.5, 7.5, 8.0, 8.5, 9.0, 9.0, 9.5, 10.10.10.10, 10.5, 10.5, 10.0, 9.0, 9.0, 9.9, 9.5, 10.10.0,10.5,11.0#
[name of registrant] Henan Jianqi medical device Co., Ltd
[residence] dingluan Industrial Zone, Changyuan County
[Tel.] 0373-8690789
[after sales service unit] Henan Jianqi medical equipment sales Co., Ltd
[name of manufacturer] Henan Jianqi Medical Equipment Co., Ltd
[residence] dingluan Industrial Zone, Changyuan County
[production address] dingluan Industrial Zone, Changyuan County
[contact information] 0373-8690789
[production license] ysyjx production license No. 20080036
[Registration Certificate No.] yxzz 20172660189
[technical requirements No.] yxzz 20172660189
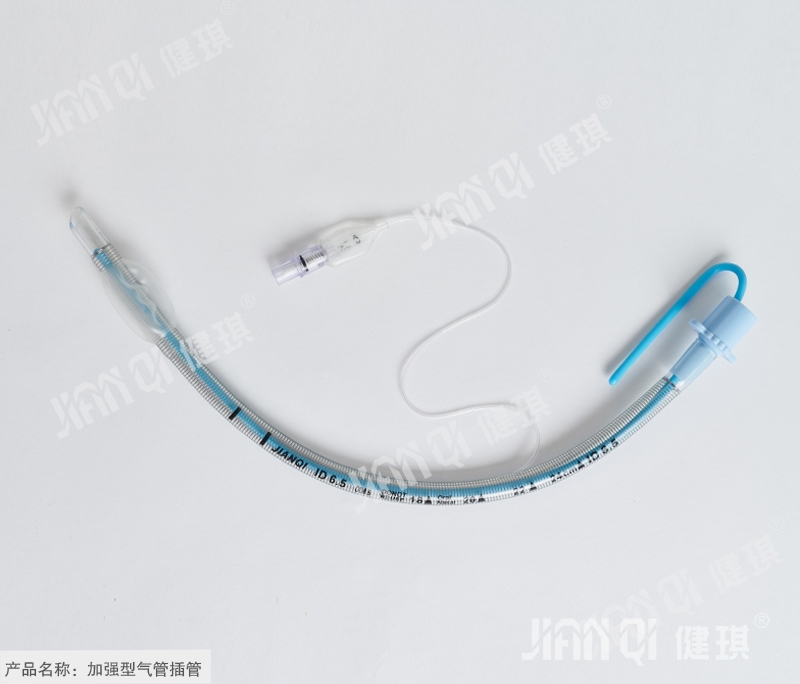
[structure and composition] this product is composed of stainless steel pipe body, cuff, joint, inflation device and guide core.
[product performance] 1. The surface of machine end joint and patient end of reinforced pipe should be neat and smooth without burr and injection flow; the extrusion of reinforced pipe body should be uniform without glue flow, and stainless steel pipe wire should be evenly attached to the middle of pipe wall. 2. The basic dimension of the reinforced pipe shall comply with the provisions of 4.1 in YY 0337.1-2002. 3. The performance of the reinforced pipe should meet the requirements of YY 0337.1-2002. 4. There should be no flattening phenomenon when the steel wire tube body is under the negative pressure of 40kpa for 15s. 5. The guide core should be clean, smooth, free of impurities and plastic. 6. The pH value of reinforcing pipe should be 6.5-7.5. 7. The product should be sterile. 8. The ethylene oxide residue of the reinforced pipe sterilized by ethylene oxide should not exceed 10 μ g / g.
[scope of application] it is used to establish artificial airway during clinical anesthesia or emergency.
[contraindications] patients with allergic product materials, laryngeal edema, acute airway inflammation, throat abscess, thoracic aortic aneurysm oppressing trachea, and severe bleeding constitution are prohibited, unless emergency endotracheal intubation is given.
[precautions] 1. This product is disposable and its package is damaged. It is forbidden to use it and destroy it after use.
2. See the seal or label of the package for the batch number and the outer packing box for the sterilization date.
3. The product was sterilized by ethylene oxide.
[usage] 1. Select the specifications and models of the patients, and open the package after confirming that the outer package is in good condition.
2. Make the patient lie on his back and check the oral cavity (take out the foreign body and removable denture, no falling tongue).
3. Using laryngoscope to lift the epiglottis to expose glottis and insert organ cannula along the side of laryngoscope.
4. Press the patient's chest, and there is air flow at the catheter mouth, indicating successful intubation.
5. After the end of use, the product should be treated as medical waste to avoid pollution.
[storage method] 1. The product should be loaded and transported in covered carriage and cabin, and kept clean. It should not be under heavy pressure, direct sunlight, rain or snow.
2. Handle with care to avoid violent collision;
3. The product should be stored away from the fire source, the relative humidity is not more than 80%, no corrosive gas and well ventilated and clean environment.

| Contact person: Ms Jin | Foreign trade: Carol Qiu |
| Phone:+86 13938450051 | |
| Landline:+86 0371 67112186 | |
 hnjq2008@163.com hnjq2008@163.com | sales@hnjianqi.com |
 www.hnjianqi.com www.hnjianqi.com | www.jianqimedical.com |
Zone, Changyuan City, Henan |