Guided by brand strategy
Based on product quality

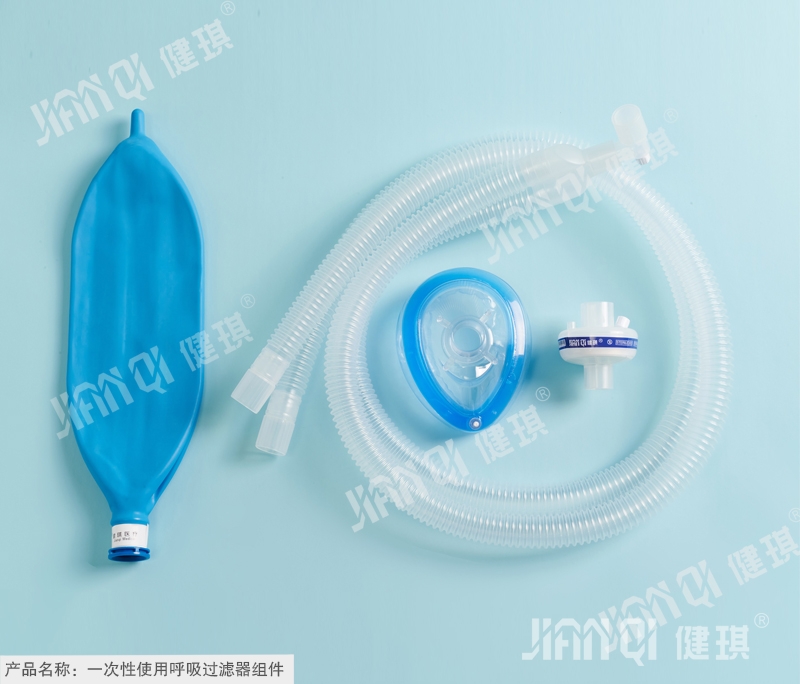
[product name] disposable respiratory filter assembly
[specification and model] respiratory pipeline type, anesthesia pipeline type
[name of registrant] Henan Jianqi medical device Co., Ltd
[address] dingluan Industrial Zone, Changyuan County, Henan Province
[Tel.] 0373-8690789
[after sales service unit] Henan Jianqi medical equipment sales Co., Ltd
[name of manufacturer] Henan Jianqi Medical Equipment Co., Ltd
[address] dingluan Industrial Zone, Changyuan County, Henan Province
[production address] dingluan Industrial Zone, Changyuan County, Henan Province
[contact information] 0373-8690789
[production license] ysyjx production license No. 20080036
[Registration Certificate No.] yxzz No. 20182080540
[structure and composition] filter package models are divided into respiratory pipeline type and anesthesia pipeline type. The respiratory pipeline type is composed of heat and moisture exchanger, disposable anesthesia breathing pipeline and nebulizer mask assembly; the anesthesia pipeline type is composed of heat and moisture exchanger, oxygen inhalation anesthesia mask and disposable anesthesia respiratory pipeline assembly.
[product performance] 1. The surface of each component shall be clean without burr and foreign matter; the contact part between mask and face shall be soft and smooth. 2. The heat and moisture exchanger shall meet the requirements of YY / T 0735.1-2009 standard or provide product qualification and inspection report. 3. Disposable anesthesia breathing pipeline should meet the requirements of yzb / Yu 0170-2010 or provide product qualification and inspection report. 4. The atomizer should be transparent. When the pressure of gas is not less than 60kpa and the gas flow rate is not less than 4L / min, the liquid in the cup can form gas mist, and the atomization amount is ≥ 0.16ml/min. 6. All parts of atomizer shall be kept smooth after connection. 7. Connection firmness: the joint of tee and bite should be firmly connected without falling off. 8. The outer surface of atomizing cup shall be provided with clear scale mark. 8. Anesthesia mask: the hook should bear a static tension of 15N, and the hook should not be broken or seriously deformed. 9. Tightness: the sealing pad of the mask is closely connected with the face and sealed well. 10. The mask should have no irritation and delayed hypersensitivity to the skin. 11. The filter bag should be sterile. 12 If the filter package is sterilized with ethylene oxide, the ethylene oxide residue shall not be greater than 10 μ g / g. 13. Cytotoxicity should be no more than grade 2.
[scope of application] it can be used as an auxiliary device for gas purification and tracheal intubation of anesthesia machine or ventilator in medical units during general anesthesia, postoperative recovery and intensive care.
[contraindications] no contraindications
[precautions] 1. This product is disposable and its package is damaged. It is forbidden to use it and destroy it after use.
2. The production batch number and expiration date are shown in the seal of the package, and the sterilization date is shown in the outer packing box.
3. This product is sterilized by ethylene oxide and has a sterility validity of two years.
[usage] 1. Check the integrity of the package before use, and do not use if the package is damaged.
2. Open the package and check whether the accessories inside are intact.
3. One section of disposable anesthesia breathing pipeline is connected to anesthesia machine or ventilator, and the other end is connected to anesthesia mask or atomizer through heat and moisture exchanger to establish a gas channel for human body.
4. After use, it should be destroyed according to the requirements of hospital or environmental protection.
[torage method] 1. The product should be loaded and transported in covered carriage and cabin, and kept clean. It should not be under heavy pressure, direct sunlight, rain or snow.
2. Handle with care to avoid violent collision;
3. The product should be stored away from the fire source, the relative humidity is not more than 80%, no corrosive gas and well ventilated and clean environment.

| Contact person: Ms Jin | Foreign trade: Carol Qiu |
| Phone:+86 13938450051 | |
| Landline:+86 0371 67112186 | |
 hnjq2008@163.com hnjq2008@163.com | sales@hnjianqi.com |
 www.hnjianqi.com www.hnjianqi.com | www.jianqimedical.com |
Zone, Changyuan City, Henan |